As clinicians, we are acutely aware that the oral biofilm accumulating daily in our patients’ mouths often compromises our best treatment efforts. Even if patients are compliant with oral hygiene at home, brushing and flossing regularly, oral biofilm still remains a source of inflammation with negative intraoral and systemic consequences.
Under the best of conditions, patients are not 100% effective in removing oral biofilm. The subjects of one clinical study left behind plaque on 85% of interdental surfaces following monitored and rigorous brushing.1 The simple use of disclosing tablets or liquid will quickly confirm the presence of plaque in neglected areas. But what about the patients who are further compromised due to medical conditions, physical infirmities, malaligned teeth, xerostomia, medication side effects, existing periodontal pockets, tobacco use, poor diet, or less-than-ideal oral hygiene? For these patients, the cumulative effects of tenacious, residual biofilm can undermine all treatment efforts, putting both patients and our meticulously crafted restorative work at risk.
The consequences of residual oral biofilm go well beyond intraoral pathology. Bacteremias can result from eating, brushing, gum chewing, toothpick use, and flossing.2–7 In the presence of oral biofilm, these bacteremias are more frequent and more severe. The aggregate systemic bacterial load resulting from multiple bacteremias causes systemic inflammation and heightens the level of inflammation in areas where systemic inflammation is already present. It is not necessary to have copious bleeding for bacteremias to be dangerous. The daily cumulative effects of very minor insults to soft tissue can result in more bacteria entering the bloodstream over time than from a single surgical procedure.2 Indeed, Richard L. Wynn, PhD, past chairman of the department of pharmacology at the University of Maryland Dental School, postulates that for those at risk for infective endocarditis, toothbrushing may pose a greater hazard than tooth extraction.8
Why is this so important? Because this is the area of greatest need—an area where we can provide critical support to patients in preventing and controlling periodontal disease. This is not just desirable adjunctive care; it is our responsibility. In a landmark article in the Journal of the American Dental Association titled “Antimicrobial mouth rinses and the management of periodontal disease,” Ira Lamster, DDS, (then dean of Columbia University’s College of Dental Medicine) emphasized the importance of daily at-home use of antimicrobial rinses by stating, “Helping patients to control their oral microbial biofilm is a primary responsibility of dental professionals.”9
Chlorhexidine gluconate is a poor candidate as an antimicrobial rinse for chronic use. Although it is efficacious, it has several negative sequelae: staining, alteration of taste, buildup of bacterial resistance, and increasing numbers of sensitivity reactions. Most importantly, it is only permitted for short-term use due to its high alcohol content and potential carcinogenicity.
Povidone iodine is efficacious and modestly priced with a substantial long-term effect, but its potential for staining and off-taste are limiting factors.
Both chlorine dioxide and sodium chlorite oral rinses can be used for daily, long-term rinsing and would provide adequate results. These rinses are more expensive than other choices, have moderate efficacy, and have limited substantivity. Some are sold directly to consumers in stores.
Molecular iodine oral rinses utilize the same biocidal form of iodine that is present in povidone iodine (molecular iodine) but in higher concentrations. At the same time, the other nonbiocidal forms of iodine are suppressed. This results in strongly efficacious, high-performance rinses with excellent safety profiles that do not stain and are pleasantly flavored (cinnamon or mint). They are ideally suited for long-term, daily use at home and are available in three forms: a ready-to-use rinse, a concentrate for subgingival irrigation with an oral irrigating device, and a gel for at-home tray application.
Povidone iodine, chlorhexidine gluconate, and sodium chlorite rinses shown in the table are generally available from dental suppliers. Molecular iodine oral rinses are available directly from ioTech International (iotechinternational.com), and activated chlorine dioxide rinse is available directly from OraCare (oracareproducts.com).
The patients in your practice who would benefit from daily, at-home use of an antimicrobial rinse include those who are immunocompromised, are caries prone, are in need of (or undergoing) periodontal treatment, and/or have a high plaque index. In other words, most patients would be candidates for this treatment.
By making a suitable antimicrobial rinse for long-term use available for sale to your patients, you would be providing better treatment outcomes and a higher standard of oral care—all without additional chair time. As you offer better protection to patients for the investments they make in their mouths, you will also positively impact their oral and systemic health. Clinicians who offer this noninvasive, conservative care have achieved high levels of patient acceptance, patient satisfaction, and greater numbers of patient referrals.
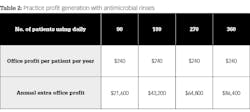
Now, let’s look at the potential financial impact to your practice when putting patients on this conservative treatment regimen. Table 2 shows potential additional practice profits one can generate by using several different patient-use scenarios.
Your patients rely on you to help them achieve and maintain optimal oral health. Providing them with antimicrobial rinses for at-home use is an important step in that direction. This conservative, effective treatment will mean improved oral health for your patients, a higher standard of care for your practice, and a substantial boost to your bottom-line profit with no additional clinician chair time required.
Disclosure: The author has a financial interest in ioTech International.
References
1. Slots J. Low-cost periodontal therapy. Periodontology 2000. 2012;60(1):110-137. doi:10.1111/j.1600-0757.2011.00429.x
2. Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117(24):3118-3125. doi:10.1161/CIRCULATIONAHA.107.758524
3. Maharaj B, Coovadia Y, Vayej AC. An investigation of the frequency of bacteraemia following dental extraction, tooth brushing and chewing. Cardiovasc J Afr. 2012;23(6):340-344. doi:10.5830/CVJA-2012-016
4. Bhanji S, Williams B, Sheller B, Elwood T, Manci L. Transient bacteremia induced by toothbrushing: a comparison of the Sonicare toothbrush with a conventional toothbrush. Pediatr Dent. 2002;24(4):295-299.
5. Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. American Heart Association. Good dental hygiene may help prevent heart infection. ScienceDaily. June 12, 2008. Accessed March 11, 2020. https://www.sciencedaily.com/releases/2008/06/080609162047.htm
6. Crasta K, Daly CG, Mitchell D, Curtis B, Stewart D, Heitz-Mayfield LJA. Bacteraemia due to dental flossing. J Clin Periodontol. 2009;36(4):323-332. doi:10.1111/j.1600-051X.2008.01372.x
7. Poveda-Roda R, Jiménez Y, Carbonell E, Gavaldá C, Margaix-Muñoz MM, Sarrión-Pérez G. Bacteremia originating in the oral cavity. A review. Med Oral Patol Oral Cir Bucal. 2008;13(6):E355-E62.
8. Wynn RL. Endocarditis-causing bacteria in the bloodstream after toothbrushing. Gen Dent. 2009;57(1):7-9.
9. Lamster IB. Antimicrobial mouthrinses and the management of periodontal diseases. J Am Dent Assoc. 2006;137(suppl 3):5S-9S. doi:10.14219/jada.archive.2006.0407