The accepted means of screening has always been visual examination and palpation. However, this has been shown to be ineffective in reducing the morbidity and mortality rates of oral cancer - especially oral squamous cell cancer, the most common type. FDA-approved, adjunctive screening tests have now been developed to better visualize early abnormal cellular changes before they can be detected with unaided visual examination.
by Herbert I. Bader, DDS, FACD, FICD
For the past decade, those of us in dentistry have been part of the growing understanding of the relationship between oral diseases, specifically periodontitis, and many systemic entities.1 These include cardiovascular disease, pulmonary disease, ischemic stroke, preterm births, and more recently, Alzheimer’s disease.2-8 The evidence increasingly points to inflammation as the common thread.9-10 As we know, periodontal disease is an inflammatory phenomenon. The stimulation of the immune mechanism by periodontal pathogens results in the release of a plethora of intermediary metabolites, such as cytokines, interleukins, matrix metalloproteinases, and others, all of which are part of the cascade of events leading to loss of attachment and eventually resorption of supporting bone. These inflammatory mediators have been linked to specific periodontal pathogens, evidence of which has been found, for example, in atherosclerotic plaque.11 There is convincing evidence, as well, that certain types of oral bacteria are present at elevated levels in oral cancer patients.12
We are now beginning to see references in the literature linking periodontitis to cancer. A recent study, for example, of 52,000 men showed that subjects with periodontal disease had a 65 percent higher risk of developing pancreatic cancer. This was true even after statistical adjustments were made for smoking, age, and other factors.13 There are well-documented connections between inflammation and cancer going back many years.14-16 As health-care practitioners, we are faced with a common inflammatory-based infection (periodontitis) having serious systemic implications, including cancer. Today’s standard of care dictates that we recognize and offer treatment options for periodontitis and oral cancer by way of appropriate referral.
The dilemmas we face in dentistry are the appalling statistics of oral cancer, despite the fact that a routine oral cancer screening is - or should be - conducted on every patient once a year. The ADA states that only 15 percent of patients are aware of being screened by their general dentist or hygienist. Oral cancers are the only malignancies with a survival rate that has not changed significantly over the past four decades. About 30,000 Americans will be diagnosed with oral or pharyngeal cancer this year,17 which accounts for 3 percent of all cancers. This poses a baseline risk of 1/10,000. One American dies every hour from these entities. The vast majority of these (90 percent) are oral squamous cell carcinomas, with a five-year survival rate of only 54 percent.18 The five-year mortality rate for carcinoma of the tongue is only 33 percent. It is suggested that there has been little change in early detection of oral cancer between 1973 and 1996, reflecting a deficiency in professional and public education with respect to the early recognition of oral cancer. More disturbing is the increasing trend of oral cancer among older black men and younger whites.19 Twenty-five percent of oral cancer patients fall into the low-risk category, lacking the common risk factors, which further emphasizes the need for a more objective, adjunctive screening technology.
It is obvious that by the time we can visually detect a lesion with the unassisted eye, it has often undergone premalignant or neoplastic changes. The rate of cancer transformation of epithelial cells demonstrating dysplasia is as high as 42 percent,20 and the incidence of this transformation seems to be related to alterations in host immunity, inflammation, angiogenesis, and metabolism. This, too, emphasizes the importance of controlling the inflammatory response associated with periodontal disease. Although the incidence of oral cancer is 1/10,000, the incidence of premalignant lesions is much more common.
Malignancies often begin with preneoplastic lesions, such as leukoplakia (white lesions). Twenty percent of leukoplakias display cellular dysplasia.This entity represents 85 percent of oral premalignant lesions. Erythroplakia (red lesions) and erythroleukoplakia (combination of white and red lesions), generally seen in elderly males, are severely dysplastic or have undergone malignant changes 91 percent of the time. These changes may be very difficult to see in their early stages depending on where they are located in the oral cavity.21-24
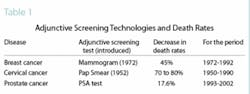
The advantage gained by adjunctive screening techniques added to the routine physical examination can be seen very clearly in Table 1. Note that the mammogram introduced in 1972 led to a decrease in death rates over the next 20 years by 45 percent. The mortality rate for cervical cancer in the 40 years following the introduction of the Pap smear went down 70 to 80 percent. It is apparent that techniques allowing for earlier detection have a significant effect on survival rates, and their value is readily accepted by all health-care professionals.
The accepted means of screening has always been visual examination and palpation. However, this has been shown to be ineffective in reducing the morbidity and mortality rates of oral cancer - especially oral squamous cell cancer, the most common type. FDA-approved, adjunctive screening tests have now been developed to better visualize early abnormal cellular changes before they can be detected with unaided visual examination.
In 2000, the Oral CDx™ oral brush biopsy test for early detection was released by CDx Laboratories Inc. (Suffern, N.Y.). The test is a minimally invasive biopsy technique for further evaluation of unexplained red or white areas in the mouth. A stiff bristle brush is rotated over the suspected area, wiped on a slide, and sealed with a solution, all supplied in single-use packages. The prepaid mailer is then sent to the laboratory where a cytopathological evaluation is done. Any dysplastic cells shown dictate the need for referral to do a biopsy. The test has been shown to be extremely sensitive and accurate in confirming the nature of apparently benign, possibly premalignant oral lesions.25-27The limitation with this technology is that it is not an early detection technique - the lesions have to be identified in order to be evaluated.
A noninvasive screening test using saliva is currently being developed. Although it appears to show great promise, it is currently limited to frank cancer, not the premalignant states. Based on work reported in 200428 at the National Institute of Dental and Craniofacial Research, scientists have identified four types of messenger RNA (mRNA) in human saliva, the presence of which seem to be predictive of a number of malignancies. This approach is in multicenter human studies at this time. One limitation of this test is that it only indicates the presence of oral cancer, not its location.
The VELscope (Visually Enhanced Lesion scope by LED Dental Inc., White Rock, B.C., Canada V4B ICS) is a device that uses tissue autofluorescence in a noninvasive technique to demonstrate the presence of premalignant or malignant lesions that may not be visible to the unaided eye.
There are about 44 studies supporting the use of VELscope, which shows high accuracy and low false-negative results. Studies show high sensitivity and specificity in this highest risk patient population, as the inclusion criteria limited the test subjects to previously diagnosed dysplasia or worse.29-32 The VELscope device costs $4,995 U.S. dollars. The LED company points out that if 1,000 patients are screened per year, the cost of the unit and disposables would be $8,000 U.S., and $3,000 thereafter.
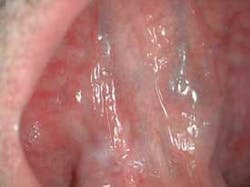
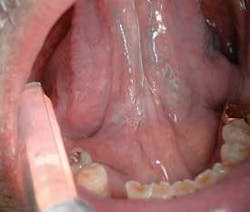
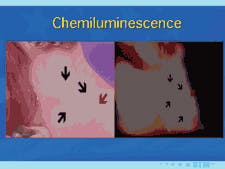
Another adjunctive system for the visualization of occult lesions uses a diffuse, nonthermogenic, chemiluminescent light source. If a suspicious lesion is indicated, it is stained with TBlue630™ (Zila® Tolonium Chloride, ZTC™). This is a patented, pharmaceutical-grade dye similar to the generic metachromatic dye known as toluidine blue. It has been shown to be an effective, accurate, and easy-to-perform technique using the ViziLite® Plus kit (Prodentec/Zila Pharmaceutical Co., Phoenix, Ariz.). Chemiluminescence has been shown to increase the visibility of subtle lesions by increasing the brightness and margins of the mucosal lesions beyond unaided visual examination.33,34
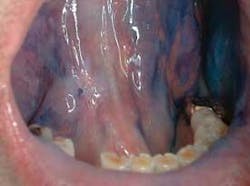
The light source is a disposable light stick contained within a retractor. The patient rinses with a flavored acetic acid solution, and then is examined. Normal epithelium absorbs the light and appears darker than abnormal epithelium, which reflects the light. As cells become dysplastic, the nuclear/cytoplasmic ratio increases. The enlarged nuclei reflect the chemiluminescent light, even from below the basement membrane. The lesion is marked on a mouth map provided in the kit, and photographed if possible. If inflammation or trauma cannot be ruled out, the patient returns in seven to 14 days. If the lesion persists, the toluidine blue (TBlue630™) dye is swabbed onto the site. Retention of the TBlue630™ indicates a need for referral for further study or biopsy. The TBlue630™ follow-up has a very high sensitivity and negative predictive value. There are very few false positives associated with this protocol. One study showed that TBlue630™ detected 16 out of 17 high-grade dysplasias.35 Other studies have indicated the extreme sensitivity of toluidine blue staining of premalignant lesions, including identification of lesions with negative biopsies that eventually converted to malignancies.36-38 The entire screening with the ViziLite® Plus kit takes less than three minutes and can be performed by a hygienist.
The ADA has developed an insurance code for adjunctive oral cancer screening (02431), which is being recognized by more and more insurance companies. The older code (D0150) covered a visual examination only. Now we can receive a fee for a much better service. The national average fee for this test is $65 to $75, with a cost to the office of only $19 per patient. Even if it is not a covered expense for the patient, the annual fee for this potentially lifesaving, two-minute test is less than the co-payment for other cancer adjunctive screens, such as mammograms, etc. If the office achieves only a 50 percent acceptance rate, which is unlikely, the typical general practice can generate an average of $40,000 annually by performing this critical screening.
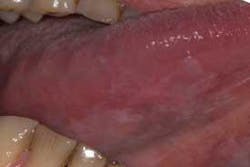
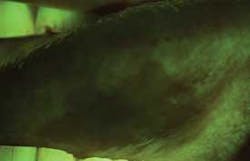
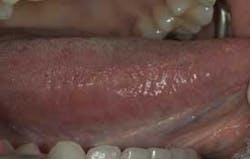
The clinical examples below show the appearance of lesions under incandescent light, chemiluminescence, and finally, the TBlue630™ stain.
The value of an adjunctive screening technique cannot be overemphasized. It is a wonderful service for our patients, it is lifesaving, and it underscores our role as health practitioners - not only for the oral cavity but for our patients’ overall health and quality of life.Visit www.dentaleconomics.com for a list of references.
Dr. Herb Bader completed his residency in periodontology at the Harvard School of Dental Medicine in 1966 and has been a lecturer in periodontology since that time. He maintains a private practice in Plymouth and Cape Cod, Mass., and lectures around the country on periodontal diagnosis and treatment. He is a frequent contributor to refereed and general dental literature. Contact Dr. Bader at [email protected] or (508) 830-3339.