Vigilant Biosciences: Real-time oral cancer detection biomarker technology
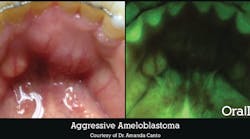
Checking for oral cancer can often be a quick assessment for bumps, swelling, ulcers, and red patches, but there’s a lot at stake. According to the American Society of Clinical Oncology, the five-year relative survival rate for mouth cancers in the US is 66%, but if diagnosed and confined within the primary site, it jumps to more than 80%.1 A new test by Vigilant Biosciences is pending FDA approval, but claims to detect oral cancers early through identification of biomarkers right in the dental office.
Groundbreaking early detection
BeVigilant OraFusion patented technology detects early-stage oral cancer by checking two biomarkers contained in saliva and a control. The company tested thousands of biomarkers over six years to settle on these. The control measures the amount of saliva and viability of the sample. The first biomarker is a transmembrane biomarker that cell membranes shed as they reproduce. It is an indicator that there is rapid reproduction of cells, a sign of cancer. The second biomarker shows the body’s attempt to suppress the activity. These biomarkers combine to measure the concentration of proteins, called proteomic biomarkers, that increase if tissue becomes cancerous. Other risk factors are taken into consideration too, such as age, tobacco and alcohol use, and gender. All the information is combined and analyzed in Vigilant Biosciences’ intelligent machine-learning software.
The science behind this is complex, but using the device is simple. Four drops of saliva are placed into a small disposable cassette that looks like a home COVID test. The cassette is then inserted into the drawer of a small reader box. Then, a report is generated that tells if cancer is likely. There’s no mailing, no long wait—the patient can be informed and referred right away. The test can be done by the dentist or hygienist, and the reader can be plugged in or battery operated so it can be easily shared between operatories.
If oral cancer is suspected, knowing whether to monitor or refer to a specialist can be complicated. Leukoplakia, lichen planus, red spots, or white spots can easily be categorized as just an oral abnormality, and in some patients, what looks innocent can quickly become life-threatening. Having a test this easy and being able to send the biomarker information to a specialist with the patient is a valuable step to getting vital treatment.
FDA Breakthrough designation
BeVigilant OraFusion was awarded FDA Breakthrough designation in March 2023. This designation identifies the product as truly unique under the current standard. FDA approval is pending, but anticipated by the summer of 2024. Henry Schein and Benco have already agreed to be distributors for this groundbreaking testing device. Already being used in Germany, Italy, and the UK, the device is awaiting final approval in India and is expected to debut shortly in the Middle East, Turkey, Thailand, Singapore, Hong Kong, and Malaysia.
Most oral cancers get diagnosed after some spread has occurred.1 Sadly, at that point, there is a much lower chance of survival. It is extremely beneficial to be able to recognize there is a need to discuss the decision or refer to a specialist while the patient is sitting in the dental chair. Just think—in 10 minutes, a dentist and/or hygienist can save a life.
Click here to learn more and be notified once available.
Reference
- Oral Health in America: Advantages and Challenges. National Institutes of Health. 2021. https://www.nidcr.nih.gov/sites/default/files/2021-12/Oral-Health-in-America-Advances-and-Challenges.pdf
Editor's note: This article originally appeared in DE Weekend, the newsletter that will elevate your Sunday mornings with practical and innovative practice management and clinical content from experts across the field. Subscribe here.