A universal approach to manage dentinal hypersensitivity and postoperative sensitivity in a restorative dental practice
The trend of using resin-based composite restorations to directly restore teeth is increasing. Many dental practices provide “mercury-free dentistry” and the placement of amalgam restorations is on the decline.1 Resin-based composites may be the material of choice for medium to large posterior restorations due to their proven wear resistance and longevity.2 Resin-based composite offers many benefits, including esthetics, minimally invasive preparation design,3 the ability to repair more predictably,4 and a strong bond to enamel. On the other hand, composite restorations have received some criticism, including the risk for secondary caries and postoperative sensitivity. When these challenges occur, it may result in additional patient visits, reduced patient satisfaction, and ultimately reduced practice efficiency and profitability. The purpose of this article is to discuss these challenges and to provide recommendations to improve long-term outcomes of resin-based composite restorations.
Literature review
When considering placing a resin-based composite restoration on a posterior tooth, there is generally a favorable long-term prognosis. Factors that may affect the longevity of the restoration are the experience level of the clinician (efficient, experienced dentists tend to provide restorations with greater longevity than students without extensive experience), the tooth being restored (restorations placed in premolar teeth generally last longer than those placed on molars due to occlusal stresses), the number of surfaces being restored (more surfaces being restored creates a greater risk for failure), the adhesive system, and the physical properties of the composite restorative material.5 It is most advantageous to follow the manufacturer’s recommendations to receive the most predictable outcomes for any dental material, particularly adhesive systems.
However, it seems that the incidence of postoperative sensitivity is not amplified by a specific adhesive system (e.g., selective- versus self-etching adhesive systems).6 Moreover, selecting a restorative material based on the application is essential for the longevity of the restoration. For example, if a restoration is planned for a tooth with a high occlusal load, selecting a composite restoration with a high fracture toughness and wear resistance is suggested. There is insufficient evidence to suggest a conclusive and consistent difference in longevity between the incremental placement of composite versus a bulk-fill composite.7
Composite failure
It has been suggested that the most reliable statistical predictor for the development of a new or secondary carious lesion is the presence of caries activity.8 Therefore, it may be inferred that patients who have been previously restored with multiple surfaces of restorations will be at a higher risk for developing caries, but the presence of restorative material does not contribute to caries risk.9 Demarco et al.10 stated that the two most prevalent causes of restorative failure of composite restorations include secondary caries and fracture. However, many long-term studies (longer than 10 years follow-up) demonstrated a higher failure rate due to fracture than to caries. He further states that many studies with five- to seven-years follow-up demonstrated a higher failure rate due to caries than fracture. He concluded that factors such as caries risk, clinical setting of the study, and socioeconomic characteristics of the population may be more reliable determinants for failure than the clinical age of a restoration.
Post-op sensitivity
Sensitivity following placement of a resin-based composite restoration seems to be individual for the patient in regard to intensity and duration, but is most likely caused by polymerization shrinkage of the composite resin or poor adaptation of the composite to dentin.11 There seems to be a positive correlation between the depth of a preparation and postoperative sensitivity.12 Moreover, there is no correlation between the method of bonding and the degree or presence of postoperative sensitivity.13,14 Dentists have opted for materials that are formulated to produce a minimal amount of polymerization shrinkage, and desensitizing agents or liners to minimize or eliminate postoperative sensitivity.
Desensitizing agents
There are many desensitizers on the dental market. Some are intended to be used during a stand-alone application on exposed root surfaces, and some are intended to be used during restorative procedures to minimize postoperative sensitivity. Newer materials have become available to offer additional benefits, including antimicrobial actions and remineralization. Future materials are being developed that will offer stress reduction, resistance to degradation, and the ability to self-repair.15 The goal for all products that are intended to reduce or eliminate post-op sensitivity is to minimize or block fluid flow at the dentin-restorative material interface by occluding dentinal tubules, coagulating or precipitating tubular fluids, encouraging secondary dentin formation, or obstructing pulpal neural response,16 without negatively impacting the physical properties of the restorative material. Arita et al.17 found that resin-based desensitizing agents offer the greatest shear bond strength and resistance to demineralization when compared to other desensitizing agents.
Desensitizer application
Desensitizers have different material properties; therefore, clinicians should take care to use them according to each manufacturer’s instructions. One desensitizer I use in my practice is Shield Force Plus (Tokuyama Dental America)—a self-etching, resin (methacrylate)-based, light-cured desensitizer that can be used alone on exposed root surfaces associated with gingival recession and in areas of erosion, or in conjunction with direct or indirect restorations. The entire application process takes 30 seconds and is ideal for the following indications: treatment of hypersensitive dentin; reduction of abrasion and erosion of exposed cervical dentin; and alleviation and/or prevention of tooth sensitivity after tooth preparation for direct and indirect restorations.
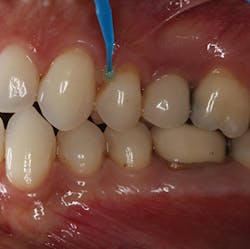
Reducing post-op sensitivity—Shield Force Plus works in two ways: by penetrating into the dentinal tubules and by forming a protective layer over the tooth surface (figure 1). When dispensed into a dappen dish, this particular material is green in color to visually aid the clinician when it is applied onto the tooth surface. During application, there is no need to rub the material into the dentinal tubules, and it should be left undisturbed for 10 seconds. The adhesive monomer of this desensitizer and the calcium in the tooth surface react. The product accumulates in the dentinal tubules and on the tooth surface.
Figure 1: Shield Force Plus is applied to the tooth with a microbrush and then left undisturbed for 10 seconds.
Manufacturer recommendations suggest applying air for 10 seconds (five seconds of gentle air and five seconds of strong air), which leaves a thin layer on the surface of the tooth (figure 2). After a 10-second light cure (figure 3), the initial green hue of the material becomes colorless. When used during a direct restorative procedure, this step would precede etching and bonding. For indirect restorative procedures, the desensitizer is applied after preparation, before making final impressions. The desensitizing ability of Shield Force Plus can last approximately three years.
Figure 2: When applying air to the tooth surface, it is important to apply gentle air pressure for five seconds and then increase the pressure for the final five seconds.
Figure 3: Once Shield Force Plus is polymerized with light, the result is a colorless layer of resin on the tooth surface.
Additional benefits—The use of a resin coating over newly prepared dentin for direct and indirect restorations has been well-described in the literature.18–20 This technique offers benefits beyond postoperative sensitivity, including improved bonding strength of resin cements and resin restorative materials, sealing marginal areas of restorations, and cavity compatibility.21,22 The added benefits are improved bond strength and marginal sealing.
Summary
In the clinical setting, it is advantageous and cost-effective to utilize materials that have multiple applications. Common complications associated with composite restorations include failure via secondary caries and immediate postoperative sensitivity. Using a desensitizer such as Shield Force Plus may minimize the incidence of postoperative sensitivity, while also enhancing bond strength to resin restorative materials and cements and providing a reliable seal at the cavosurface margin. Desensitizers can be time- and cost-efficient, especially if they minimize postoperative complications and promote long-term outcomes of direct and indirect restorations.
References
1. Lynch CD, Wilson NH. Managing the phase-down of amalgam: Part I. Educational and training issues. Br Dent J. 2013;215(3):109-113. doi: 10.1038/sj.bdj.2013.737.
2. Borgia E, Baron R, Borgia JL. Quality and survival of direct light-activated composite resin restorations in posterior teeth: A 5- to 20-year retrospective study. J Prosthodont. 2017;1-9. doi: 10.1111/jopr.12630.
3. Cenci M, Demarco F, de Carvalho R. Class II composite restorations with two polymerization techniques: relationship between microtensile bond strength and marginal leakage. J Dent. 2005;33(7):603-610. doi: 10.1016/j.jdent.2005.01.001.
4. Estay J, Martin J, Viera V, et al. 12 years of repair of amalgam and composite resins: A clinical study. Oper Dent. 2018;43(1):12-21. doi: 10.2341/16-313-C.
5. Demarco FF, Corrêa MB, Cenci MS, Moraes RR, Opdam NJ. Longevity of posterior composite restorations: not only a matter of materials. Dent Mater. 2012;28(1):87-101. doi: 10.1016/j.dental.2011.09.003.
6. Perdigão J, Swift EJ Jr. Critical appraisal: post-op sensitivity with direct composite restorations. J Esthet Restor Dent. 2013;25(4):284-288. doi: 10.1111/jerd.12045.
7. van Dijken JW, Pallesen U. Posterior bulk-filled resin composite restorations: a 5-year randomized controlled clinical study. J Dent. 2016;51:29-35. doi: 10.1016/j.jdent.2016.05.008.
8. Doméjean S, White JM, Featherstone JD. Validation of the CDA CAMBRA caries risk assessment—a six-year retrospective study. J Calif Dent Assoc. 2011;39(10):709-715.
9. Featherstone JDB, Chaffee BW. The evidence for caries management by risk assessment (CAMBRA). Adv Dent Res. 2018;29(1):9-14. doi: 10.1177/0022034517736500.
10. Demarco FF, Corrêa MB, Cenci MS, Moraes RR, Opdam NJ. Longevity of posterior composite restorations: not only a matter of materials. Dent Mater. 2012;28(1):87-101. doi: 10.1016/j.dental.2011.09.003.
11. Bhatti UA, Ahmed A, Javed Q. Frequency of postoperative sensitivity in posterior Class 1 composite restorations. Pak Oral Dent J. 2014;34(3):532-535. http://www.podj.com.pk/Aug_2014/PODJ-33.pdf.
12. Burke FJT. Technique tips—the cost of one post-operative sensitivity following placement of a posterior composite restoration. Dent Update. 2015;42(7):692-693.
13. Reis A, Dourado Loguercio A, Schroeder M, Luque-Martinez I, Masterson D, Cople Maia L. Does the adhesive strategy influence the post-operative sensitivity in adult patients with posterior resin composite restorations?: A systematic review and meta-analysis. Dent Mater. 2015;31(9):1052-1067. doi: 10.1016/j.dental.2015.06.001.
14. Perdigão J, Swift EJ Jr. Critical appraisal: post-op sensitivity with direct composite restorations. J Esthet Restor Dent. 2013;25(4):284-288. doi: 10.1111/jerd.12045.
15. Fugolin APP, Pfeifer CS. New resins for dental composites. J Dent Res. 2017;96(10);1085-1091. doi: 10.1177/0022034517720658.
16. Trushkowsky RD, Oquendo A. Treatment of dentin hypersensitivity. Dent Clin North Am. 2011;55(3):599-608. doi: 10.1016/j.cden.2011.02.013.
17. Arita S, Suzuki M, Kazama-Koide M, Shinkai K. Shear bond strengths of tooth coating materials including experimental materials contained various amounts of multi-ion releasing fillers and their effects for preventing dental demineralization. Odontology. 2017;105(4):426-436. doi: 10.1007/s10266-016-0290-1.
18. Magne P. IDS: Immediate dentin sealing (IDS) for tooth preparations. J Adhes Dent. 2014;16(6):594. doi: 10.3290/j.jad.a33324.
19. Qanungo A, Aras MA, Chitre V, Mysore A, Amin B, Daswani SR. Immediate dentin sealing for indirect bonded restorations. J Prosthodont Res. 2016;60(4):240-249. doi: 10.1016/j.jpor.2016.04.001.
20. Brigagão VC, Barreto LFD, Goncalves KAS, et al. Effect of interim cement application on bond strength between resin cements and dentin: Immediate and delayed dentin sealing. J Prosthet Dent. 2017;117(6):792-798. doi: 1016/j.prosdent.2016.09.015.
21. Nikaido T, Tagami J, Yatani H, et al. Concept and clinical application of the resin-coating technique for indirect restorations. Dent Mater J. 2017:1-5. doi: 10.4012/dmj.2017-253.
22. Turkistani A, Sadr A, Shimada Y, Nikaido T, Sumi Y, Tagami J. Sealing performance of resin cements before and after thermal cycling: evaluation by optical coherence tomography. Dent Mater. 2014;30(9):993-1004. doi: 10.1016/j.dental.2014.05.010.
Pamela Maragliano-Muniz, DMD, BSDH, a graduate of Tufts University School of Dental Medicine, obtained her certificate in advanced prosthodontics from UCLA School of Dentistry. An international lecturer and author, she teaches at Tufts and serves on the editorial board for DentistryIQ’s Product Navigator e-newsletter. A recipient of numerous leadership awards, her practice was awarded the ADA’s 2010 Adult Preventive Care Practice of the Year. She practices in Salem, Massachusetts.